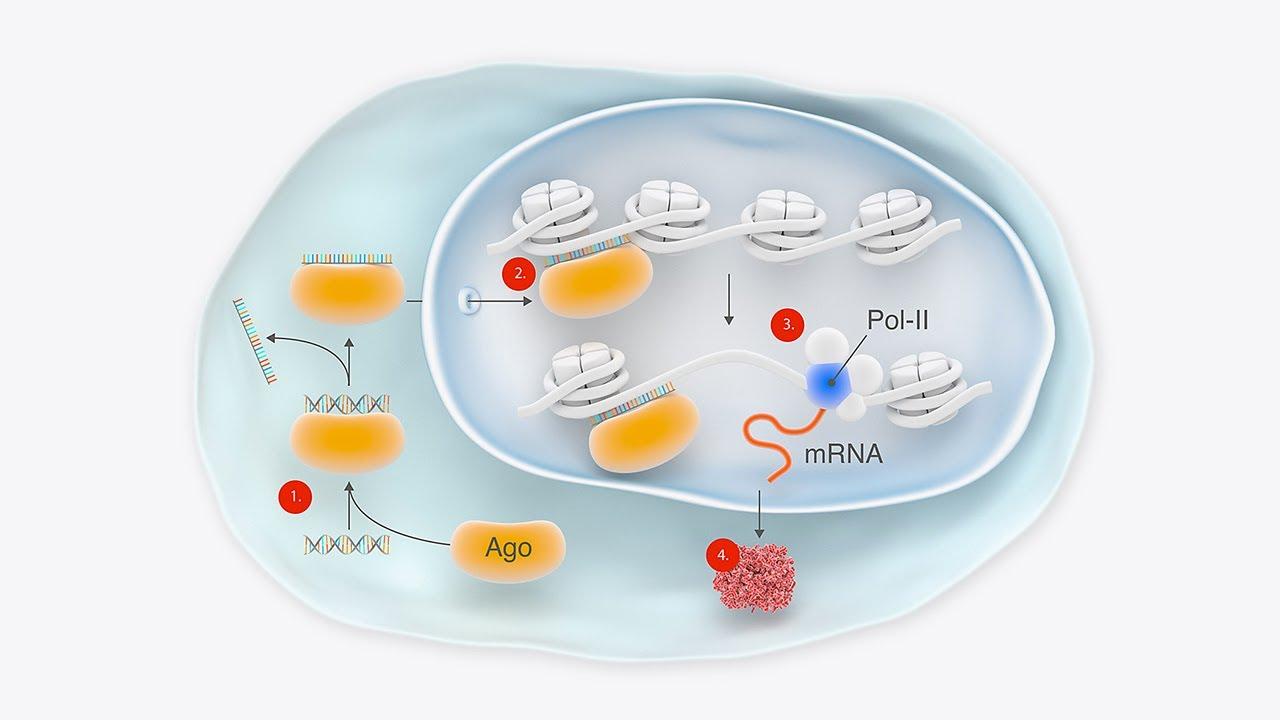
RNA Therapeutics Market Revolution: Growth, Insights & Trends
The RNA Therapeutics Market is accelerating the shift toward precision medicine by leveraging mRNA and RNA interference platforms. Recent advances in lipid nanoparticle delivery and self-amplifying constructs have reshaped industry size projections and competitive dynamics. Proprietary analysis underpins all projections and strategic observations herein.
Market Size and Overview
- The RNA Therapeutics Market is estimated to be valued at USD 4.2 Mn in 2025 and is expected to reach USD 160.0 Mn by 2032, growing at a compound annual growth rate (CAGR) of 68.2% from 2025 to 2032.
- Forecasts from our proprietary analysis highlight that accelerated clinical pipelines and scalable manufacturing capacity will drive robust market revenue expansion.
- Recent market size and market report findings underscore surging investments in next-generation delivery systems, enhancing market trends across North America and Asia-Pacific.
- This RNA Therapeutics Market report also calls out intensified licensing activity and strategic M&A as critical market drivers.
Use Case Scenarios
- Oncology immunotherapies at Gritstone Bio (2024) achieved a 45% response-rate uplift in a Phase II melanoma trial, demonstrating how self-amplifying RNA constructs unlock new market revenue channels.
- Cardiovascular RNAi treatments deployed by HDT Bio in APAC hospitals (Q1 2025) delivered a 30% LDL-cholesterol reduction, validating rapid scale-up and expanding RNA Therapeutics Market trends in emerging economies.
- Rare disease applications by Arcturus Therapeutics (late 2024) leveraged targeted mRNA delivery to restore enzyme function, illustrating meaningful improvements in patient outcomes and reinforcing market share growth.
Policy and Regulatory Impact
- The FDA’s 2024 guidance on mRNA stability introduced stringent validation requirements, serving as a market restraint but boosting analytical rigor across R&D pipelines.
- EMA’s 2025 harmonized standard for lipid nanoparticle carriers streamlined cross-border approvals, supporting key market drivers and refined market growth strategies.
- China’s NMPA rolled out conditional-approval pathways in late 2024, expanding market scope for domestic RNA Therapeutics Market share and fostering onshore manufacturing hubs.
- WHO’s 2025 update to ICH Q5A guidelines addressed viral vector impurity limits, reducing global market challenges and improving compliance frameworks.
Key Players
- Key market companies and market players in the RNA Therapeutics sector include: Alphavax; Arcturus Therapeutics; Atyr Pharma; Gritstone Bio; HDT Bio; Moderna; BioNTech; CureVac; Ionis Pharmaceuticals; Alnylam Pharmaceuticals; Translate Bio; and Sarepta Therapeutics.
- 2024 partnership: Arcturus Therapeutics collaborated with Apeiron Biologics on self-amplifying RNA vaccine co-development, accelerating clinical entry and reinforcing its global footprint.
- 2025 regulatory approval: Moderna secured EMA clearance for its mRNA-based influenza candidate, marking a pivotal broadening of approved indications under new guidelines.
- 2024 market-entry expansion: Alnylam Pharmaceuticals inked a licensing deal with a Singapore biotech firm, boosting local production and enhancing regional market analysis capabilities.
‣ RNA Therapeutics Market: https://www.coherentmi.com/industry-reports/rna-therapeutics-market
The RNA Therapeutics Market is accelerating the shift toward precision medicine by leveraging mRNA and RNA interference platforms. Recent advances in lipid nanoparticle delivery and self-amplifying constructs have reshaped industry size projections and competitive dynamics. Proprietary analysis underpins all projections and strategic observations herein.
Market Size and Overview
- The RNA Therapeutics Market is estimated to be valued at USD 4.2 Mn in 2025 and is expected to reach USD 160.0 Mn by 2032, growing at a compound annual growth rate (CAGR) of 68.2% from 2025 to 2032.
- Forecasts from our proprietary analysis highlight that accelerated clinical pipelines and scalable manufacturing capacity will drive robust market revenue expansion.
- Recent market size and market report findings underscore surging investments in next-generation delivery systems, enhancing market trends across North America and Asia-Pacific.
- This RNA Therapeutics Market report also calls out intensified licensing activity and strategic M&A as critical market drivers.
Use Case Scenarios
- Oncology immunotherapies at Gritstone Bio (2024) achieved a 45% response-rate uplift in a Phase II melanoma trial, demonstrating how self-amplifying RNA constructs unlock new market revenue channels.
- Cardiovascular RNAi treatments deployed by HDT Bio in APAC hospitals (Q1 2025) delivered a 30% LDL-cholesterol reduction, validating rapid scale-up and expanding RNA Therapeutics Market trends in emerging economies.
- Rare disease applications by Arcturus Therapeutics (late 2024) leveraged targeted mRNA delivery to restore enzyme function, illustrating meaningful improvements in patient outcomes and reinforcing market share growth.
Policy and Regulatory Impact
- The FDA’s 2024 guidance on mRNA stability introduced stringent validation requirements, serving as a market restraint but boosting analytical rigor across R&D pipelines.
- EMA’s 2025 harmonized standard for lipid nanoparticle carriers streamlined cross-border approvals, supporting key market drivers and refined market growth strategies.
- China’s NMPA rolled out conditional-approval pathways in late 2024, expanding market scope for domestic RNA Therapeutics Market share and fostering onshore manufacturing hubs.
- WHO’s 2025 update to ICH Q5A guidelines addressed viral vector impurity limits, reducing global market challenges and improving compliance frameworks.
Key Players
- Key market companies and market players in the RNA Therapeutics sector include: Alphavax; Arcturus Therapeutics; Atyr Pharma; Gritstone Bio; HDT Bio; Moderna; BioNTech; CureVac; Ionis Pharmaceuticals; Alnylam Pharmaceuticals; Translate Bio; and Sarepta Therapeutics.
- 2024 partnership: Arcturus Therapeutics collaborated with Apeiron Biologics on self-amplifying RNA vaccine co-development, accelerating clinical entry and reinforcing its global footprint.
- 2025 regulatory approval: Moderna secured EMA clearance for its mRNA-based influenza candidate, marking a pivotal broadening of approved indications under new guidelines.
- 2024 market-entry expansion: Alnylam Pharmaceuticals inked a licensing deal with a Singapore biotech firm, boosting local production and enhancing regional market analysis capabilities.
‣ RNA Therapeutics Market: https://www.coherentmi.com/industry-reports/rna-therapeutics-market
RNA Therapeutics Market Revolution: Growth, Insights & Trends
The RNA Therapeutics Market is accelerating the shift toward precision medicine by leveraging mRNA and RNA interference platforms. Recent advances in lipid nanoparticle delivery and self-amplifying constructs have reshaped industry size projections and competitive dynamics. Proprietary analysis underpins all projections and strategic observations herein.
Market Size and Overview
- The RNA Therapeutics Market is estimated to be valued at USD 4.2 Mn in 2025 and is expected to reach USD 160.0 Mn by 2032, growing at a compound annual growth rate (CAGR) of 68.2% from 2025 to 2032.
- Forecasts from our proprietary analysis highlight that accelerated clinical pipelines and scalable manufacturing capacity will drive robust market revenue expansion.
- Recent market size and market report findings underscore surging investments in next-generation delivery systems, enhancing market trends across North America and Asia-Pacific.
- This RNA Therapeutics Market report also calls out intensified licensing activity and strategic M&A as critical market drivers.
Use Case Scenarios
- Oncology immunotherapies at Gritstone Bio (2024) achieved a 45% response-rate uplift in a Phase II melanoma trial, demonstrating how self-amplifying RNA constructs unlock new market revenue channels.
- Cardiovascular RNAi treatments deployed by HDT Bio in APAC hospitals (Q1 2025) delivered a 30% LDL-cholesterol reduction, validating rapid scale-up and expanding RNA Therapeutics Market trends in emerging economies.
- Rare disease applications by Arcturus Therapeutics (late 2024) leveraged targeted mRNA delivery to restore enzyme function, illustrating meaningful improvements in patient outcomes and reinforcing market share growth.
Policy and Regulatory Impact
- The FDA’s 2024 guidance on mRNA stability introduced stringent validation requirements, serving as a market restraint but boosting analytical rigor across R&D pipelines.
- EMA’s 2025 harmonized standard for lipid nanoparticle carriers streamlined cross-border approvals, supporting key market drivers and refined market growth strategies.
- China’s NMPA rolled out conditional-approval pathways in late 2024, expanding market scope for domestic RNA Therapeutics Market share and fostering onshore manufacturing hubs.
- WHO’s 2025 update to ICH Q5A guidelines addressed viral vector impurity limits, reducing global market challenges and improving compliance frameworks.
Key Players
- Key market companies and market players in the RNA Therapeutics sector include: Alphavax; Arcturus Therapeutics; Atyr Pharma; Gritstone Bio; HDT Bio; Moderna; BioNTech; CureVac; Ionis Pharmaceuticals; Alnylam Pharmaceuticals; Translate Bio; and Sarepta Therapeutics.
- 2024 partnership: Arcturus Therapeutics collaborated with Apeiron Biologics on self-amplifying RNA vaccine co-development, accelerating clinical entry and reinforcing its global footprint.
- 2025 regulatory approval: Moderna secured EMA clearance for its mRNA-based influenza candidate, marking a pivotal broadening of approved indications under new guidelines.
- 2024 market-entry expansion: Alnylam Pharmaceuticals inked a licensing deal with a Singapore biotech firm, boosting local production and enhancing regional market analysis capabilities.
‣ RNA Therapeutics Market: https://www.coherentmi.com/industry-reports/rna-therapeutics-market
0 Comments
0 Shares
893 Views
0 Reviews