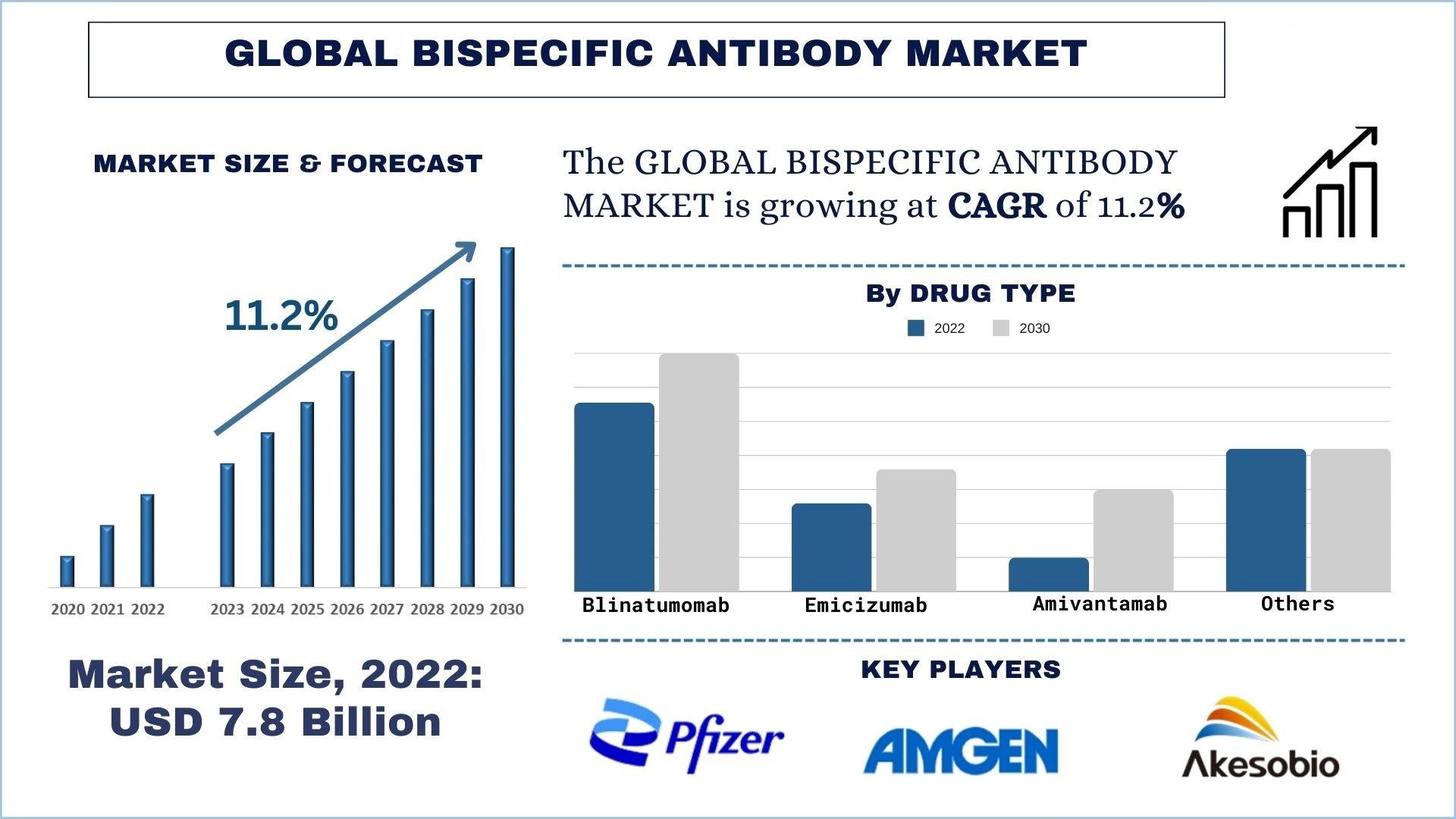
According to the UnivDatos Market Insights, the surge in cases of chronic diseases like cancer and hemophilia and the rise in research and development activities will drive the global scenario of the bispecific antibody market and as per their “Bispecific Antibody Market” report, the global market was valued at USD 7.8 Billion in 2022, growing at a CAGR of about 11.2% during the forecast period from 2023 - 2030 to reach USD billion by 2030. The bispecific antibody market in North America is excelling at a tremendous rate for several reasons. Clinical trials for bispecific antibodies are conducted to evaluate their safety, efficacy, pharmacokinetics, pharmacodynamics, and optimal dosing regimens across various disease indications. Clinical trials for bispecific antibodies play a crucial role in establishing their safety and efficacy, informing clinical practice guidelines, and advancing therapeutic innovation in various disease indications. These trials are conducted according to rigorous ethical and regulatory standards to ensure patient safety and data integrity. Here are some detailed aspects of clinical trials for bispecific antibodies:
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=56911
Phase I Trials: Phase I trials are typically the first-in-human studies designed to assess the safety, tolerability, and pharmacokinetics of bispecific antibodies. These trials involve dose escalation to determine the maximum tolerated dose (MTD) or recommended phase II dose (RP2D). Phase I trials also provide preliminary data on efficacy and pharmacodynamic effects.
Phase II Trials: Phase II trials aim to further evaluate the safety and preliminary efficacy of bispecific antibodies in specific patient populations or disease indications. These trials often involve larger patient cohorts and may include randomized controlled trials (RCTs) to compare bispecific antibodies with standard of care or other treatment modalities. Phase II trials also provide additional data on pharmacokinetics, pharmacodynamics, and dose-response relationships.
Phase III Trials: Phase III trials are large-scale, randomized, controlled trials conducted to confirm the safety and efficacy of bispecific antibodies and support regulatory approval for marketing authorization. These trials typically involve a larger number of patients recruited across multiple clinical sites and may include comparative effectiveness studies against standard of care or placebo. Phase III trials provide robust evidence of clinical benefit and are essential for regulatory approval.
Special Population Studies: Clinical trials for bispecific antibodies may include special population studies to evaluate their safety and efficacy in specific patient groups, such as pediatric patients, elderly patients, patients with comorbidities, or patients with rare diseases. These studies help to assess the applicability of bispecific antibodies across diverse patient populations.
Combination Therapy Trials: Bispecific antibodies may be evaluated in combination with other therapies, such as chemotherapy, radiation therapy, targeted therapy, or immunotherapy, in clinical trials. Combination therapy trials aim to assess the potential synergistic effects, safety, and efficacy of bispecific antibodies when used in conjunction with other treatment modalities.
Biomarker Studies: Clinical trials for bispecific antibodies may incorporate biomarker studies to identify predictive biomarkers of response, resistance, or toxicity. Biomarker analyses help to elucidate the underlying mechanisms of action, optimize patient selection criteria, and personalize treatment approaches.
Post-Marketing Surveillance: After regulatory approval, post-marketing surveillance studies may be conducted to monitor the long-term safety, effectiveness, and real-world use of bispecific antibodies in clinical practice. These studies provide additional data on rare or long-term adverse events and inform post-approval risk management strategies.
Notable recent clinical trials involving bispecific antibody are:
In December 2023, Abramson Cancer Center at Penn Medicine initiated a research study to test the safety and effectiveness of the experimental drug mosunetuzumab or obinutuzumab and glofitamab when given after CAR (genetically modified) T cells.
In May 2022, Merus N.V. initiated a study of bispecific antibody MCLA-145 in patients with advanced or metastatic malignancies.
In July 2021, JW Pharmaceutical initiated a clinical trial to evaluate the safety, efficacy, and pharmacokinetics of FVIII Inhibitor titers of Hemlibra subcutaneous injection in Korean Hemophilia A patients with/without FVIII Inhibitors.
Upright and Steady Climb: The bispecific antibody has already made its mark in the market. As this dynamic market continues to develop and grow, it provides a ray of hope for the global effort to develop advanced therapeutics. The healthcare domain is constantly innovating and redefining its innovative system from the ground up.
Click here to view the Report Description & TOC https://univdatos.com/report/bispecific-antibody-market/
Conclusion:
The bispecific antibody research is still in its early stages, due to the rapid development and expansion of therapeutic advancement. This is indicative of the ongoing efforts to improve healthcare infrastructure and access globally, which is gradually changing the landscape. Furthermore, the increased investment in research and development in the healthcare sector is further increasing the potential of the market. Despite the unique challenges it faces, the world is making progress towards developing more effective therapeutics. As this nascent market continues to grow and develop, it has the potential to contribute significantly to global efforts to combat many of the conditions associated with it.
Contact Us:
UnivDatos Market Insights
Email - contact@univdatos.com
Contact Number - +1 9782263411
Website - https://univdatos.com/