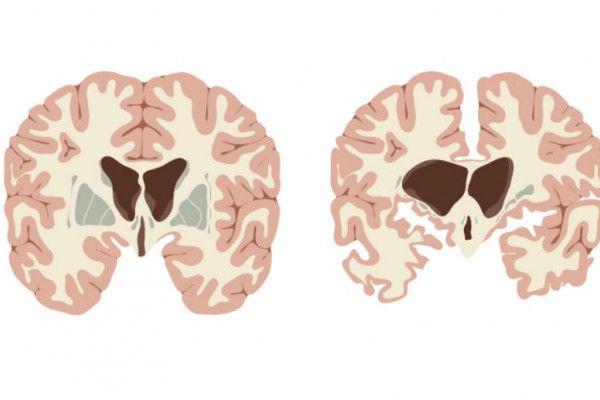
Huntington’s Disease (HD) is a neurodegenerative disorder characterized by progressive motor dysfunction, cognitive decline, and psychiatric symptoms. It is caused by a mutation in the huntingtin gene, leading to the degeneration of nerve cells in certain areas of the brain. Despite being a rare disease, affecting approximately 5-10 people per 100,000, its impact is profound on individuals and families. However, the landscape of HD treatment is evolving rapidly, with promising advancements anticipated in the coming decade.
Market Dynamics:
The market for treatments for Huntington's disease (ハンチントン病治療市場) was projected to be valued US$ 360 million in 2021 and is projected to expand at a compound annual growth rate (CAGR) of 20% between 2022 and 2032.
By the end of 2032, the market is projected to reach US$ 2.7 billion.
The market for treatments for Huntington's disease (HD) is anticipated to expand over the forecast period, with a projected global market value of US$ 432 million by the end of 2022.
Get Free Sample Research Report Copy:
https://www.factmr.com/connectus/sample?flag=S&rep_id=7121
Huntington’s Disease Treatment Market Key Players:
· H. Lundbeck A/S
· Bausch Health Companies Inc.
· Hetero Drugs
· Lupin Limited
· Hikma Pharmaceuticals Plc.
· Dr. Roddy’s Laboratories Ltd.
· Sun Pharmaceutical Industries Ltd.
Huntington’s Disease Treatment Market Segmentation:
· By Treatment
o Symptomatic Treatment of Huntington’s disease
o Disease-Modifying Therapies for Huntington’s disease
· By Region
o North America
o Latin America
o Europe
o APAC
o MEA
Key Drivers:
- Advancements in Genetic Research: With the advent of precision medicine and gene-editing technologies like CRISPR-Cas9, there is growing optimism for targeted therapies aimed at correcting the underlying genetic mutation responsible for HD.
- Neuroprotective Strategies: Research efforts are focused on identifying compounds that can protect neurons from degeneration, thereby slowing disease progression. Neurotrophic factors and small molecule drugs targeting specific pathways show promising results in preclinical studies.
- Improved Diagnostic Tools: Early and accurate diagnosis is crucial for effective management of HD. The development of novel biomarkers and advanced imaging techniques enables clinicians to detect the disease in its early stages, facilitating timely intervention and personalized treatment approaches.
- Collaborative Initiatives: Public-private partnerships and collaborations between academia, pharmaceutical companies, and patient advocacy groups are accelerating the pace of drug development and clinical trials, fostering a conducive environment for innovation in HD therapeutics.
Treatment Landscape:
- Disease-Modifying Therapies: Several disease-modifying therapies are in various stages of clinical development, targeting different aspects of HD pathophysiology such as protein aggregation, mitochondrial dysfunction, and inflammation. These include antisense oligonucleotides, gene silencing approaches, and novel protein modulators.
- Symptomatic Management: While disease-modifying treatments aim to alter the course of the disease, symptomatic management remains a cornerstone of HD care. Pharmacological interventions targeting motor symptoms, psychiatric manifestations, and cognitive impairment help improve patients' quality of life and functional independence.
- Non-Pharmacological Interventions: Complementary therapies such as physical therapy, speech therapy, occupational therapy, and counseling play a crucial role in addressing the multidimensional needs of HD patients and their caregivers. Assistive devices and adaptive technologies further aid in enhancing mobility and communication.
Browse Full Report @ https://www.factmr.com/report/huntingtons-disease-treatment-market
Challenges and Opportunities:
- Clinical Trial Design: Designing clinical trials for neurodegenerative diseases like HD poses unique challenges due to the heterogeneous nature of the patient population, variable disease progression, and lack of reliable biomarkers. Innovative trial designs and outcome measures are essential to ensure the efficacy and safety of investigational therapies.
- Access to Treatment: Access to novel therapies remains a concern, particularly in resource-limited settings and underserved communities. Efforts to streamline regulatory processes, expand healthcare infrastructure, and implement equitable pricing strategies are imperative to ensure equitable access to life-changing treatments.
- Patient Engagement: Engaging patients and caregivers in the drug development process is crucial to ensure that research priorities align with their needs and preferences. Patient advocacy organizations play a pivotal role in amplifying the voices of the HD community and driving policy changes that prioritize patient-centered care.
𝐂𝐨𝐧𝐭𝐚𝐜𝐭:
US Sales Office :
11140 Rockville Pike
Suite 400
Rockville, MD 20852
United States
Tel: +1 (628) 251-1583
E-Mail: sales@factmr.com