Software as a Medical Device Market Size, Share, Growth, Trends and Forecast 2024-2032
Introduction:
Software as a Medical Device (SaMD) refers to software specifically designed for one or more medical purposes that operates independently, without being integrated into any hardware medical device. SaMD applications include functions such as diagnosing, monitoring, or managing health conditions. This software runs on general-purpose computing platforms, meaning it does not require a physical medical device to fulfill its medical functions. It can be utilized across various platforms, including mobile applications and cloud-based systems, which further enhance its accessibility and usability in healthcare systems.
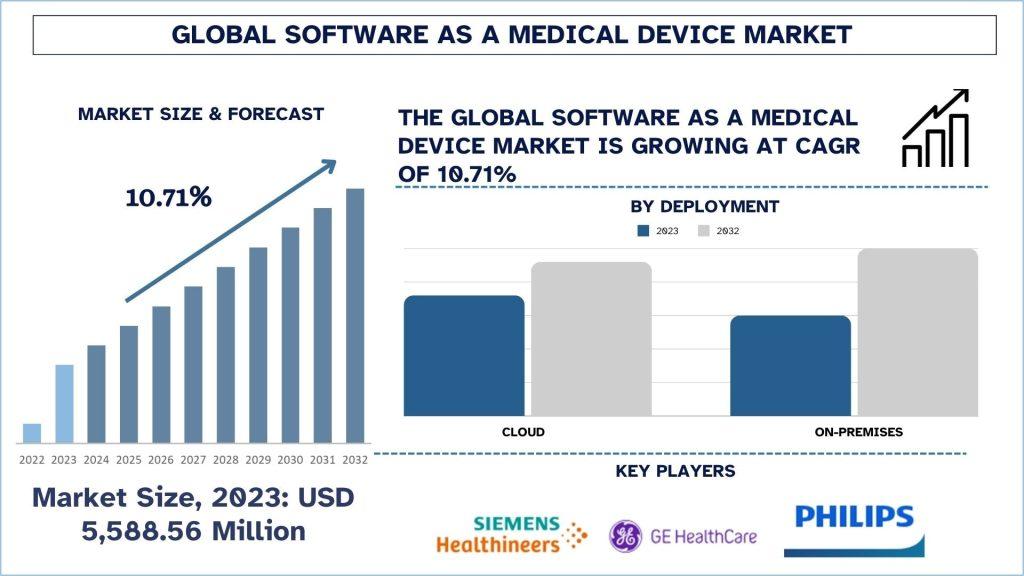
According to the Univdatos Market Insights analysis, growing investments in SaMD for improving healthcare delivery services, increasing research and development activities and growing mergers and acquisitions in this industry will drive this market growth. As per their “Software as a Medical Device Market” report; the global market was valued at USD 5,588.56 million in 2023, growing at a CAGR of 10.71% during the forecast period from 2024 - 2032.
Request Free Sample Pages with Graphs and Figures Here - https://univdatos.com/get-a-free-sample-form-php/?product_id=66644
Software as a Medical Device Market Overview in North America:
North America Software as a Medical Device market holds a significant market share in 2023. Some of the factors attributed to the growth are increasing number of aging population, rising prevalence of chronic diseases, well-developed healthcare infrastructure, along with higher number of companies operating in the region such as Qure.ai, Imagen Technologies, Inc., etc. Additionally, the region has also focused on consistently developing innovative SaMD solutions for various medical purposes, that has led to increasing number of FDA approvals within the region. For instance, In January 2024, Qure.ai, a U.S-based leading global manufacturer of medical imaging solutions, announced its recent FDA clearance for AI-enabled SaMD solutions for radiography and medical imaging analysis. These products are highly efficient in detecting cancer. These frequent US FDA approvals are driving growth of North America SaMD market. With the expansion of the SaMD solution in the region as well as collaboration among the leading market players in the region, the market is anticipated to rise in the forthcoming years.
Growing Demand and Industry Trends:
One of the key factors that have promoted the demand for software as a medical device market in North America is growing adoption of digital healthcare system in this region. With growing internet connection and digitalization, more people are using smartphones and wearables to self-monitor their diseases with the help of SaMD installed in their digital devices. Additionally, SaMD applications by healthcare professionals in the healthcare settings provide them with ease of remote monitoring, which is rising demand for SaMD solutions.
Government initiatives such as increased emphasis on developing strong digital health action plans is essential for enhancing healthcare access and quality. For instance, according to WHO “Global Strategy on Digital Health 2020-2025” report, each country needs to have a strong digital health action plan to bring access to quality health services. It is of prime importance to strengthen digital health action plans by focusing on implementation of AI and machine learning within the healthcare system. This would enhance accuracy in diagnosis and ensure quality and improved medical treatment. Such initiatives can further drive demand for software as a medical device in the healthcare sector.
Related Reports-
Antibiotic Resistance Market: Current Analysis and Forecast (2024-2032)
Opioid Use Disorder (OUD) Treatment Market: Current Analysis and Forecast (2024-2032)
Prospects and Opportunities:
The integration of software as a medical device solution with Artificial Intelligence (AI) is another crucial factor that contributes to the increased adoption of such solutions. North America is well-equipped with advanced healthcare infrastructure and solutions, which act as a major factor for increased adoption of AI-based SaMD solutions in this region. AI-based SaMD assists healthcare professionals in quick data gathering and data interpretation and thus saves operating time, which is leading to huge demand for AI-based SaMD products among the healthcare professionals. For instance, in March 2024, FDA published an article on how artificial intelligence and medical products are working together efficiently and contributing to elevating the overall healthcare industry. This article focuses on the importance and benefits of developing AI-based SaMD action plans to provide improved patient care. Considering these technological advancements, there is significant opportunities in software as a medical device market in North America in the forthcoming years.
For more information about this report visit- https://univdatos.com/report/software-as-a-medical-device-market/
Conclusion:
The North America software as a medical device market is at its peak with these supportive factors of the health infrastructure, government investment, industry collaboration, and technological innovation. As the region continues strengthening its health sector and its effectiveness through investment, increasing regulatory frameworks, and strategic partnerships, it will be in good stead to face challenges and benefits emerging in the space of the software as a medical device market.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


