Graves Disease Market Analysis, Report, Trends & Forecast
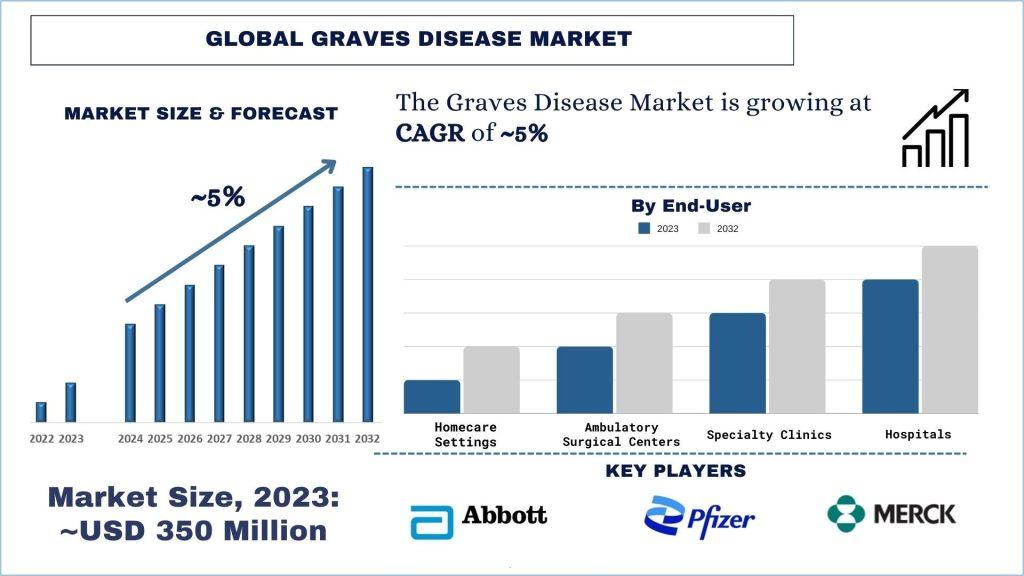
According to the UnivDatos Market Insights analysis, growing government initiatives to improve healthcare infrastructure and increase awareness about thyroid disorders are driving market growth in the region will drive the scenario of global graves disease and as per their “Global Graves Disease Market” report, the market was valued at USD ~350 billion in 2023, growing at a CAGR of ~5% during the forecast period from 2024 – 2032.
Graves’ disease is a common autoimmune disease affecting the thyroid gland, it remains a concern to the patient and healthcare givers in the United States. Since Graves’ disease is mainly manifested by symptoms that display serious complications with adequate treatment, it is necessary to create a comprehensive approach.
Graves Disease Treatment Demand in the U.S.
Graves’ disease is as of now prevalent in the United States, and its frequency is growing, just as the frequencies of autoimmune disorders all over the world increase. Graves’ disease has been on the rise within the United States of America, due to various reasons including; increased population, and hereditary and environmental causes. Over time, knowledge about thyroid diseases has raised the number of people suffering from Graves’ disease, which in turn increases the need for proper diagnostics and the development of individual therapy regimens.
Treatment Options
The options for treating Graves’ disease in the U.S are widely available and provide patients with numerous options to successfully cope up with their disease. The most popular drugs for the treatment of thyrotoxicosis include methimazole and propylthiouracil because they block the synthesis of thyroid hormones and reduce the symptoms. Another treatment is radioactive iodine therapy where radioactive iodine is taken in order to kill the overactive thyroid cells. This though may lead to hypothyroidism especially if thyroid gland is absent or damaged, necessitating lifelong thyroid hormone replacement therapy.
Thyroidectomy or surgical treatment is only recommended where other treatment modes cannot be used or have not yielded the desired results. This involves the surgical obliteration of the thyroid gland either partially or in its entirety and has been found to alleviate the symptoms of Graves’ disease significantly.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=60473
Advancements
Technological advancements have significantly influenced the global graves disease market in North America.
· The Food and Drug Administration (FDA) approved for Priority Assessment a new drug application (NDA) for TransConTM PTH (palopegteriparatide) in adults diagnosed with hypoparathyroidism in November 2022.
· In May 2023, Amolyt Pharma disclosed an oral presentation at the 25th European Congress of Endocrinology (ECE) 2023. The presentation emphasizes the potential benefits of eneboparatide as a therapy for hypoparathyroidism, a condition in which numerous people have or are susceptible to acquiring osteopenia and osteoporosis.
· Ascendis Pharma has announced the launch of its second TransCon™ product, YORVIPATH, now available in Germany and Austria for the treatment of adults with chronic hypoparathyroidism. YORVIPATH leverages Ascendis Pharma's innovative TransCon technology, designed to improve patient outcomes by providing a sustained release of active ingredients, thereby enhancing efficacy and convenience.
Government Regulations
The officials of the United States have the responsibility of overseeing the care and management of Graves’ disease. The drugs and medications for treating Graves’ disease are approved by the Food and Drug Administration (FDA), and they guarantee the safety of the medications and therapies. Furthermore, CMS controls payment policies for treatment; this authority aims to make it possible for patients to receive care at an affordable cost.
Over time the U. S. government has made an effort to increase access to care for patients with Graves’ disease. The Patient Protection and Affordable Care Act (ACA) also covers more people through health insurance and embraces coverage of basic health services, including prescription drugs and preventive care services. This has eliminated the issue of financial constraints in the availability of care for patients with Graves’ disease hence, the ability of the patients to get the right kind of care for the treatment of the disease.
Conclusion
The Graves disease market in the U.S. is evolving rapidly, driven by factors such as the increasing prevalence of the condition and advancements in treatment options. With a focus on personalized medicine and patient-centered care, healthcare providers in the U.S. are working diligently to ensure that individuals affected by Graves' disease receive the best possible care. Moving forward, policymakers and healthcare stakeholders need to continue to collaborate to improve access to care, enhance treatment options, and ultimately, improve outcomes for patients with Graves' disease in the U.S.
Contact Us:
UnivDatos Market Insights
Email - contact@univdatos.com
Contact Number - +1 9782263411
Website -www.univdatos.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Jogos
- Gardening
- Health
- Início
- Literature
- Music
- Networking
- Outro
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


