Pipette Tips Market Report, Share, Trends & Forecast
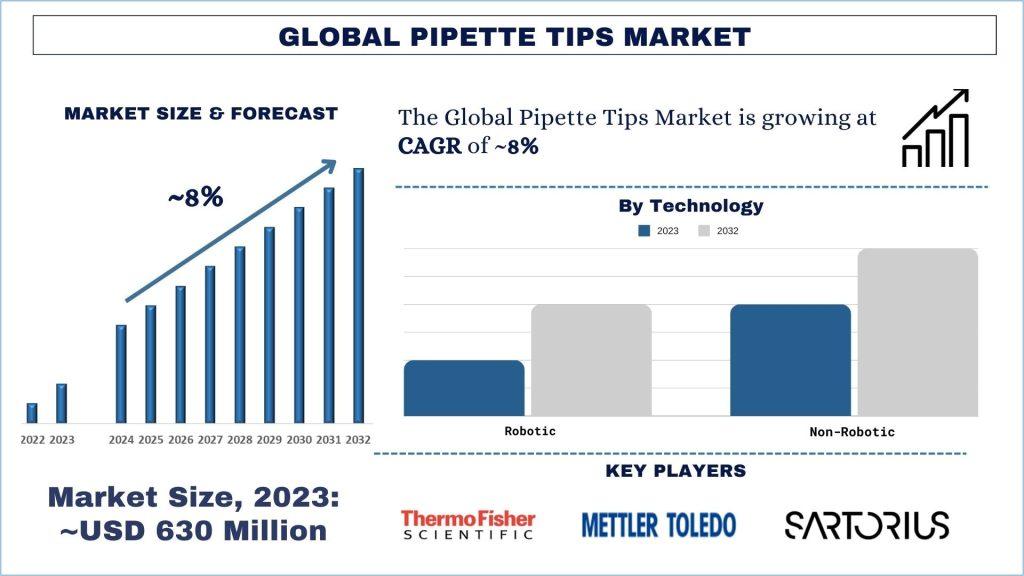
According to the UnivDatos Market Insights analysis, the high incidence of chronic diseases boosts the need for diagnostic tests, and increasing pipette tip usage will drive the scenario of the global pipette tips as per their “Pipette Tips Market” report, the market was valued at USD ~630 million in 2023, growing at a CAGR of ~8% during the forecast period from 2024 – 2032.
The United States remains a pivotal market for the global pipette tips industry, characterized by robust research and development activities, advanced healthcare infrastructure, and stringent regulatory frameworks. Pipette tips, essential tools for precise liquid handling in laboratories, play a crucial role in various scientific, clinical, and industrial applications across the country.
Demand Drivers
This demand for pipette tips in the U. S. is because of having many diagnosis centers, pharmaceutical laboratories, and innovations in biomedical engineering. Pipette tips enable proper handling of samples found in clinical laboratories hence enhancing the reliability of the outcomes necessary for patient management. In addition, conditions like Chronic diseases/Infectious outbreaks, including currently affecting the world COVID-19, have significantly raised the need for diagnostics testing, thereby increasing the overall consumption of pipette tips.
Technological Innovations
The pipette tips market is continuously developing due to technological progress, especially in the United States, which leads to scientific discoveries in the field of research and development with companies such as Biolite, Pipette.com, and Sorenson BioScience. Robotic systems have contemporary influenced the laboratory environment where high throughput screening and personalized medicine projects are now easily achievable. Automated pipette tips are made to give efficient results, thereby lowering contamination rates and enhancing the reliability of experiments catering to the needs of pharmaceutical and clinical research.
Advancements in materials science have supported advances in such pipette tips as low-retention pipette tips, and filter pipette tips. High-tip-angle suggestions reduce equilibration contact with the vial sidewall, which enables greater sample recovery as well as precise volume detection.
Regulatory Landscape
Regulations made by the government of the U. S determine how pipette tips should be manufactured, the channel of distribution as well as their usage to ensure that only quality products that meet the regulatory standards are used. The FDA categorizes pipette tips as Class I medical devices because they are considered low risk to patients and lab personnel whereas the tips that pipettes are made for a particular use are classified into certain classes. The FDA requirements such as GMP give a clearance or approval to the manufacturers who are willing to market pipette tips in the United States.
Apart from federal regulations some states or municipalities, for example, may have their standards regarding lab practices and the use of consumables, including pipette tips. This way the pipette tips are abiding by the industry norms set by the CLSI and the ISO also guarantees that they have met certain quality and performance status.
Access sample report (including graphs, charts, and figures): https://univdatos.com/get-a-free-sample-form-php/?product_id=61501
Market Challenges
Quality Assurance and Standardization
Pipette tips may be manufactured by different companies to different standards depending on which brand of pipette they are designed for or the type; this makes quality and performance standardization critically important if results are to be consistent and legally admissible the range of pipette tips that call for standardization includes those manufactured by different companies for different brands of pipettes or different types of pipettes. Different tips could affect the consistency and comparability of experiments when using tips in laboratories, due to the differences in their design and the properties of the materials used to form tips and other approaches employed in manufacturing the tips. Laboratories need to use common reference systems and methods that will enable them to assess and select the pipette tips that would be most suitable for kinds of applications.
Sustainability Initiatives
New concepts involving environmental sustainability are inspiring the desire for green pipette tip substitutes in the U. S. At the same time, laboratories are researching more sustainable materials to replace the existing plastic pipette tips, because disposal of plastic waste remains a major problem. Companies are therefore rising to the challenges brought about by the sustainability trends through innovation of environmentally friendly products and realizing sustainable practices in their product chain.
Conclusion
Other factors enhancing the growth of the U. S. pipette tips market include technological development, changes in laws and acts related to the manufacturing of the products, as well as changes in the healthcare sector. It may be concluded that with developing new laboratory experiments and increasing attention towards bringing more accurate, efficient, and eco-friendly tips for experimental procedures, there is a constant potential for pipette tips. Players in the pipette tips market see openings to enter new markets including individualized medicine, digitalization, and possibilities linked to disease control as well as foodborne disease outbreaks which define the future of the pipette tips market in the USA.
Contact Us:
UnivDatos Market Insights
Email - contact@univdatos.com
Contact Number - +1 9782263411
Website -www.univdatos.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


