Dental Implants And Prosthetics Contract Manufacturing Market Role in Scaling for OEM Growth
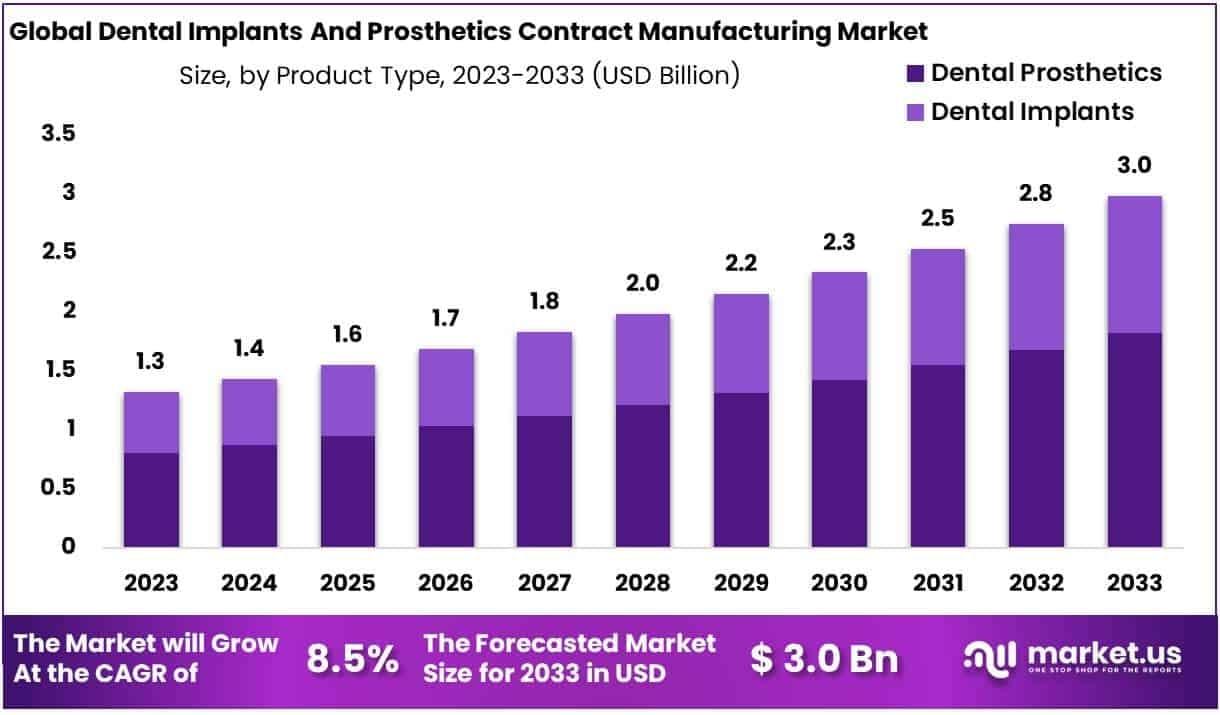
The Global Dental Implant and Prosthetic Contract Manufacturing Market size is expected to be worth around USD 3.0 Billion by 2033 from USD 1.3 Billion in 2023, growing at a CAGR of 8.5% during the forecast period from 2024 to 2033.
By 2025, the Dental Implants and Prosthetics Contract Manufacturing Market is evolving into a scale-driven, compliance-oriented powerhouse. Manufacturers are deploying automated milling and multi-axis machining to produce large volumes of implant components with micron-level accuracy. Factory workflows employ in-line surface finishing and laser marking to meet batch-testing luxury standards, while regulatory scrutiny ensures consistency through FDA and EU MDR compliance.
These production lines support both emerging dental brands and global OEMs seeking reliable supply chains across North America, Europe, and Asia. As practices consolidate and demand for aesthetic implants grows, contract manufacturers focused on scalable, compliant, and cost-effective production are becoming strategic partners in dental innovation.
Click here for more information: https://market.us/report/dental-implant-and-prosthetic-contract-manufacturing-market/
Key Market Segments
Product Type
Dental Implants
-
- Titanium Implants
- Zirconium Implants
Dental Prosthetics
-
- Bridges & Crowns
- Dentures
- Abutments
End-use
-
- Medical Device Companies
- Dentistry Companies
Emerging Trends
- Multi-axis CNC milling enabling high-volume implant components.
- Automated post-processing—daylight sintering and laser etching—for finish consistency.
- Regulatory-heavy workflows aligned with FDA and EU MDR standards.
- Regional contract networks offering redundancy and global footprint.
Use Cases
- A CMO produces 10,000 identical titanium screws monthly with sub-10µm tolerances.
- Batch-sintered zirconia crowns emerge from dual-stage post-processing lines.
- European lab renovates quality control to meet EU MDR; U.S. affiliate supports FDA audits.
- A North American practice sources implants via regional CMOs with backup production in Mexico.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


