Regulatory Affairs Outsourcing Market Growth, Size, Revenue Analysis, Top Leaders and Forecast 2030
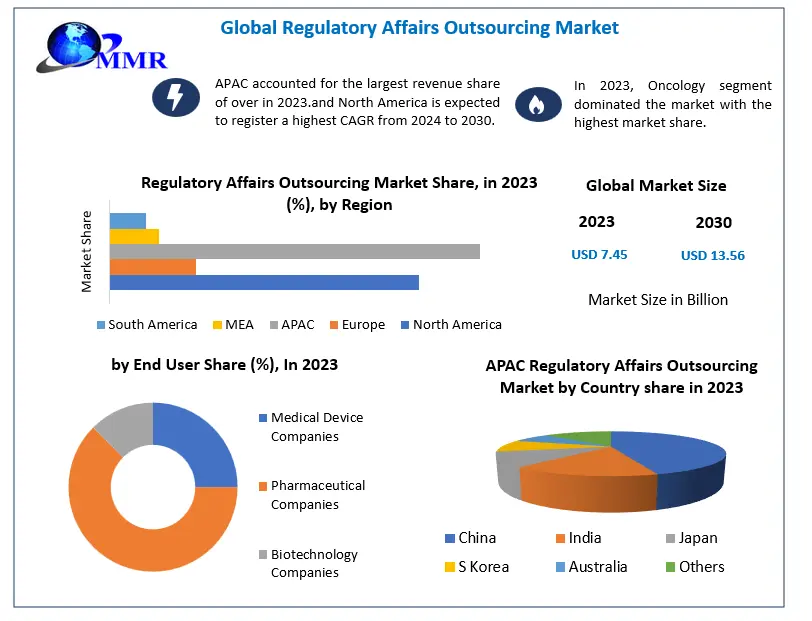
Regulatory Affairs Outsourcing Market is poised for substantial growth, with projections indicating an expansion from USD 7.77 billion in 2025 to approximately USD 15.93 billion by 2029. This growth trajectory, marked by a compound annual growth rate (CAGR) of 14.0%, is driven by the increasing complexity of regulatory submissions, digital transformation in regulatory processes, and a heightened focus on compliance and risk management.
Market Overview
Regulatory affairs outsourcing involves delegating regulatory compliance and documentation responsibilities to external specialized service providers. This practice is prevalent in industries such as pharmaceuticals, biotechnology, medical devices, and food and beverages, where adherence to stringent regulatory standards is paramount. Outsourcing regulatory affairs functions allows companies to leverage specialized expertise, ensuring timely product approvals and facilitating market access.
To Know More About This Report Request A Free Sample Copy: https://www.maximizemarketresearch.com/request-sample/44332/
Key Market Drivers
1. Increasing Complexity of Regulatory Submissions
The evolving regulatory landscape and the growing intricacy of regulatory submissions are prompting companies to seek external expertise to navigate complex compliance requirements efficiently.
2. Digital Transformation in Regulatory Processes
The adoption of digital technologies in regulatory affairs is streamlining processes, enhancing efficiency, and reducing the time required for regulatory approvals, thereby driving the demand for outsourcing services.
3. Emphasis on Compliance and Risk Management
With the rising importance of compliance and risk management in product development and market entry, companies are increasingly outsourcing regulatory affairs to ensure adherence to global standards and mitigate potential risks.
Market Segmentation
By Service Type:
- Regulatory Strategy and Consulting: Providing guidance on regulatory pathways and strategies.
- Regulatory Submissions Management: Handling the preparation and submission of regulatory documents.
- Regulatory Affairs Outsourcing: Managing ongoing regulatory compliance and documentation tasks.
- Others: Including regulatory training and market authorization support.
By End-User Industry:
- Pharmaceuticals and Biotechnology: Navigating complex regulatory requirements for drug development and approval.
- Medical Devices: Ensuring compliance with regulatory standards for medical device manufacturing and marketing.
- Food and Beverages: Adhering to food safety and labeling regulations.
- Others: Including cosmetics and consumer health products.
Regional Insights
North America:
Dominates the global regulatory affairs outsourcing market due to the presence of major pharmaceutical and biotechnology companies, stringent regulatory standards, and a high demand for specialized regulatory services.
Europe:
Experiences steady growth driven by stringent regulatory frameworks, an aging population, and a strong focus on healthcare innovation.
Asia-Pacific:
Anticipated to witness the fastest growth owing to expanding pharmaceutical and biotechnology industries, increasing regulatory complexity, and a growing emphasis on compliance.
Competitive Landscape
The regulatory affairs outsourcing market is characterized by the presence of several key players focusing on strategic collaborations, technological advancements, and expansion into emerging markets to strengthen their market position.
Major Manufacturers:
- Parexel International Corporation
- Covance Inc.
- Charles River Laboratories International, Inc.
- PPD, Inc.
- IQVIA Holdings Inc.
Future Outlook
The regulatory affairs outsourcing market is set to experience significant growth, driven by the increasing complexity of regulatory requirements, digital transformation in regulatory processes, and a heightened focus on compliance and risk management. As companies seek to navigate the evolving regulatory landscape efficiently, outsourcing regulatory affairs functions will play a crucial role in ensuring timely product approvals and facilitating global market access.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


