Comprehensive Guide to Methyl Vinyl Ketone (MVK): Properties, Applications, and Safety
What is Methyl Vinyl Ketone?
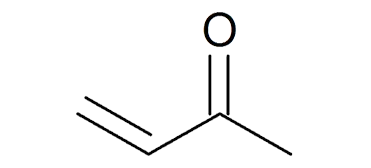
Methyl Vinyl Ketone (MVK) is an organic compound with the formula CH₂=CHCOCH₃, classified as an α,β-unsaturated ketone. It is a colorless, volatile liquid with a sharp, acrid odor. Owing to its high reactivity, particularly toward nucleophiles, MVK is a pivotal compound in organic synthesis and industrial manufacturing processes.
Chemical Structure and Properties of Methyl Vinyl Ketone
- Molecular Formula: C₄H₆O
- Molar Mass: 70.09 g/mol
- IUPAC Name: But-3-en-2-one
- Appearance: Colorless liquid
- Odor: Pungent and sharp
- Boiling Point: 81°C (177.8°F)
- Density: 0.840 g/cm³
- Solubility: Soluble in water and organic solvents
- Vapor Pressure: High at room temperature
The molecular structure includes a vinyl group (–CH=CH₂) conjugated with a carbonyl group (C=O), making it susceptible to Michael additions and other nucleophilic attacks. This dual reactivity enables it to function as a building block in various chemical syntheses.
Industrial Production of Methyl Vinyl Ketone
Methyl Vinyl Ketone is industrially produced through:
- Dehydrogenation of Methyl Ethyl Ketone (MEK)
- MEK is passed over a catalyst at high temperatures to yield MVK and hydrogen.
- Condensation of Acetone and Formaldehyde
- A base-catalyzed aldol condensation, followed by dehydration, forms MVK.
Due to its toxicity and volatility, industrial production is carefully regulated with advanced ventilation systems and safety protocols.
Applications of Methyl Vinyl Ketone in Industry
1. Polymer and Resin Manufacturing
MVK is widely used as a co-monomer in the production of polymers and resins. Its high reactivity allows it to copolymerize with styrene, acrylates, and methacrylates, resulting in materials with improved adhesion, flexibility, and chemical resistance.
2. Organic Synthesis Intermediate
In organic synthesis, MVK serves as a key intermediate for the preparation of:
- Pharmaceutical compounds
- Pesticides and agrochemicals
- Flavors and fragrances
- Specialty chemicals
Its electrophilic double bond enables it to participate in Michael addition reactions, Diels–Alder cycloadditions, and other functional group transformations.
3. Fine Chemicals and Specialty Applications
MVK is involved in synthesizing:
- Vitamin A intermediates
- Prostaglandin analogs
- Photoinitiators for UV-curing systems
- Crosslinking agents in adhesives
Its ability to form complex cyclic and acyclic structures makes it invaluable in research laboratories and industrial R&D settings.
Health and Safety Considerations
Toxicological Profile
Methyl Vinyl Ketone is classified as toxic by inhalation, skin contact, and ingestion. Exposure to MVK can cause:
- Severe eye and respiratory tract irritation
- Dermatitis on skin contact
- CNS depression at high concentrations
- Pulmonary edema in severe cases
Occupational Exposure Limits
- OSHA PEL (Permissible Exposure Limit): 0.5 ppm
- NIOSH REL (Recommended Exposure Limit): 0.5 ppm
- IDLH (Immediately Dangerous to Life or Health): 5 ppm
Handling and Storage Guidelines
- Store in a cool, well-ventilated area, away from heat and ignition sources.
- Use only with appropriate personal protective equipment (PPE): chemical-resistant gloves, eye protection, and respiratory protection.
- Avoid prolonged or repeated exposure.
- Employ closed systems or local exhaust ventilation when handling the compound.
Environmental Impact and Regulatory Status
MVK is classified under multiple regulatory frameworks due to its toxicity and environmental hazards:
- Listed on TSCA (Toxic Substances Control Act) Inventory
- Subject to EPA regulations under the Clean Air Act due to its status as a hazardous air pollutant (HAP)
- Requires strict disposal protocols in compliance with RCRA hazardous waste guidelines
Despite its reactivity, MVK is biodegradable under aerobic conditions, but its acute aquatic toxicity demands careful containment and waste treatment.
Conclusion
Methyl Vinyl Ketone is a highly reactive and valuable compound in industrial chemistry and organic synthesis. Its versatility across multiple industries, including pharmaceuticals, adhesives, and polymers, highlights its significance. However, due to its toxicity and environmental concerns, stringent safety measures and regulatory compliance are essential during handling, storage, and disposal. Sulfur trioxide pyridine is widely used as a sulfonating agent in organic synthesis due to its high reactivity and controlled handling.
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


