MASH as the New Frontier: Moving Beyond NASH in Liver Disease Therapy
Understanding the Shift from NASH to MASH
The field of liver disease has undergone a significant transformation with the reclassification of Nonalcoholic Steatohepatitis (NASH) as Metabolic Dysfunction-Associated Steatohepatitis (MASH). This change better reflects the disease’s metabolic origins, emphasizing its strong links to obesity, diabetes, and metabolic syndrome. While Non-Alcoholic Fatty Liver Disease (NAFLD) encompasses a broad spectrum of conditions, MASH specifically highlights the inflammatory and fibrotic progression of liver damage. The transition from NASH to MASH is more than a name change—it represents a refined understanding of the disease and its treatment strategies.
MASH Treatment Landscape: Prevalence and Market Growth
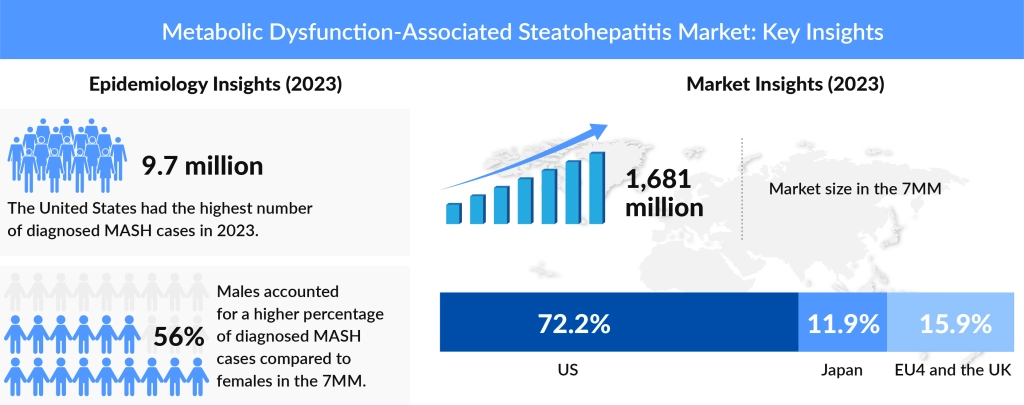
The prevalence of MASH is rising at an alarming rate, affecting millions worldwide. Previously studied under NASH epidemiology, the disease remains a major health concern due to its potential progression to cirrhosis and hepatocellular carcinoma. The market for NASH treatment had already been expanding due to increasing awareness and diagnosis rates. With the transition to MASH, pharmaceutical companies are recalibrating their research efforts. Analysts expect sustained market growth, with FDA approvals playing a key role in advancing treatment options.
Clinical Trials for MASH Treatment: Pipeline Advancements
The clinical trials for MASH treatment are progressing rapidly, with numerous investigational therapies in different phases of development. The NASH treatment landscape has expanded to include promising drug candidates targeting metabolic dysfunction, fibrosis, and inflammation. Many pharmaceutical companies are actively working on breakthrough treatments, with several late-stage therapies moving closer to FDA approval.
The Transition to MASH: A Shift in Research and Development
With the, pharmaceutical companies are refining their clinical trial methodologies to focus more on metabolic dysfunction. This shift is expected to enhance drug efficacy, improve patient outcomes, and facilitate regulatory approval. By aligning therapeutic strategies with the metabolic underpinnings of MASH, researchers aim to develop more effective and targeted treatments.
Challenges and Opportunities in MASH Drug Development
Despite significant progress, challenges remain, including disease variability, the absence of standardized biomarkers, and regulatory complexities. However, the transition from NASH to MASH also presents new opportunities for pharmaceutical advancements. The demand for better NASH treatment options is driving innovation in precision medicine, paving the way for more effective, personalized therapies.
Conclusion
The reclassification of Nonalcoholic Steatohepatitis (NASH) to MASH marks a crucial milestone in liver disease research and treatment. With ongoing clinical trials for MASH treatment and the emergence of new therapies, the future of liver disease management is evolving. As pharmaceutical innovations continue, FDA-approved drugs will be instrumental in shaping the treatment landscape and improving patient outcomes globally.
Latest Reports Offered By Delveinsight
Attention Deficit Hyperactivity Disorder Market | Lactose Intolerance Market | Urea Cycle Disorders Market | Overactive Bladder Syndrome Market | Surgical Energy Instruments Market | Pipeline Assessment Services | Penile Cancer Market | Total Knee Arthroplasty Market | Indwelling Catheters Market
About Delveinsight:
DelveInsight is a leading provider of market research and consulting services, specializing in the life sciences and healthcare industries. Our insights support pharmaceutical, biotechnology, and medical device companies in navigating competitive environments and achieving long-term success.
Contact Information: Kanishk
Email: kkumar@delveinsight.com
- Art
- Causes
- Crafts
- Dance
- Drinks
- Film
- Fitness
- Food
- Games
- Gardening
- Health
- Home
- Literature
- Music
- Networking
- Other
- Party
- Religion
- Shopping
- Sports
- Theater
- Wellness


